- 0571-8536 0887
- 13588470430
- jension@hzsunchem.com
- Language:English
- English

pd_meltingpoint:223 °C (dec.)(lit.)
Appearance:white powder
Purity:99%
|
Adrenocorticotropic hormone drugs |
Hydrocortisone acetate is an adrenal hormone drugs, white crystalline powder, odorless, bitter taste. The melting point is 216~222 ℃, right optical. Slightly soluble in ethanol, ether, chloroform, acetone, insoluble in water. It was synthesized by In 17α-hydroxy progesterone. Hydrocortisone acetate has important physiological activities such as anti-inflammatory, anti-viral, anti-allergic, anti-shock, reducing inflammatory exudation effect, commonly used in clinical adrenal insufficiency and autoimmune diseases. Preparations are injection, tablets, ointments and eye drops. Adding a small amount of hydrocortisone acetate in some cosmetics cortisol can increase the efficacy of anti-allergy and improve the degree of whitening of the skin, but long-term using of such substances can cause the skin produce hormone-dependent disease. Our cosmetics cosmetics specifications prescribedthathormone substances can not be added. |
|
Indications |
For allergic, non-infectious skin diseases and a number of proliferative skin disorders. Such as dermatitis, eczema, neurodermatitis, seborrheic dermatitis and itching psychosis. Figure 1 the chemical structure formula of tetra-fluoroethane. |
|
Contraindications |
1. This medicine and matrix components allergy and allergic to other glucocorticoids are prohibited. 2. The primary bacterial, fungal and viral infectious skin diseases such as disabledare prohibited. The above information is edited by the lookchem of KuiMing. |
|
Adverse Reactions |
Long-term using can cause local skin atrophy, telangiectasia, pigmentation, folliculitis, perioral dermatitis and secondary infection. |
|
Precautions |
1. Not long-term, large-scale use. 2. The coated parts such as a burning sensation, itching, swelling, etc., should stop the medication. |
|
Chemical Property |
White or almost white crystalline powder, odorless. Mp218-221.5 ℃; 25D + 166 ° (dioxane) Specific rotation [α]; ethanol maximum absorption at 240nm wavelength. Insoluble in water, slightly soluble in ethanol (1: 230) and chloroform (1: 150), insoluble in ether. |
|
Production methods |
Hydrocortisone and acetic anhydride in pyridine esterification. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Aldesleukin: avoid concomitant use. Antibacterials: metabolism accelerated by rifampicin; metabolism possibly inhibited by erythromycin; concentration of isoniazid possibly reduced. Anticoagulants: efficacy of coumarins and phenindione may be altered. Antiepileptics: metabolism accelerated by carbamazepine, phenobarbital, fosphenytoin, phenytoin and primidone. Antifungals: increased risk of hypokalaemia with amphotericin - avoid; metabolism possibly inhibited by itraconazole and ketoconazole. Antivirals: concentration possibly increased by ritonavir. Ciclosporin: rare reports of convulsions in patients on ciclosporin and high-dose corticosteroids. Cobicistat: concentration of hydrocortisone possibly increased - increased risk of adrenal suppression. Diuretics: enhanced hypokalaemic effects of acetazolamide, loop diuretics and thiazide diuretics. Vaccines: high dose corticosteroids can impair immune response to vaccines - avoid concomitant use with live vaccines. |
|
Purification Methods |
The acetate recrystallises from Me2CO/Et2O or aqueous Me2CO as hygroscopic monoclinic crystals. UV has max at 242 nm (A1cm 1% 390) in MeOH. Its solubility at 25o is: H2O (0.001%), EtOH (0.45%), MeOH (0.04%), Me2CO (1.1%), CHCl3 (0.5%), Et2O (0.15%), and it is very soluble in Me2NCHO. [Wendler et al. J Am Chem Soc 74 3630 1952; Antonucci et al. J Org Chem 18 7081 1953, Beilstein 8 IV 3424.] |
|
Brand name |
Cortef Acetate (Pharmacia & Upjohn); Cortifoam (Schwarz Pharma); Cortril (Pfizer); Dricort (Ingram); Hydrocortone (Merck). |
InChI:InChI=1/C23H32O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h10,16-18,20,26,28H,4-9,11-12H2,1-3H3/t16?,17?,18-,20?,21-,22-,23-/m0/s1
The invention provides a dehalogenation ...
The invention relates to a preparation p...
The present invention involves improved ...
-

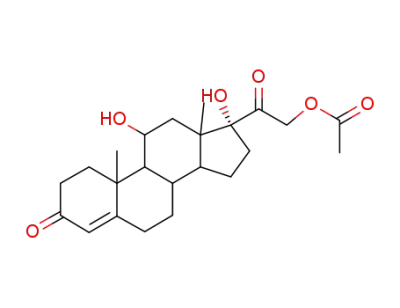
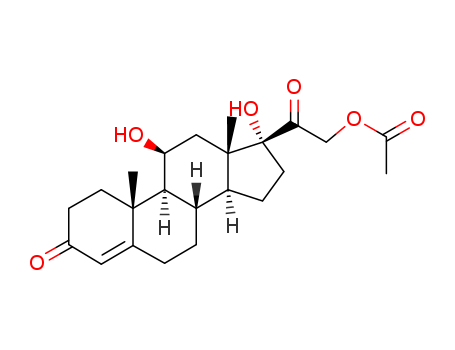
Hydrocortison-acetat
| Conditions | Yield |
|---|---|
|
21,21-Diiod-11β,17α-dihydroxy-pregnen-84)-dion-(3,20), H2O, AcOH-haltiges Aceton, K-Acetat;
|
|
|
Verb. C21H30O5, Ac2O, Pyridin;
|
|
|
Hydrocortison-acetat-(17), Bzl., p-Toluolsulfonsaeure (wenig), Δ;
|
|
|
Cortisol, Pyridin, Ac2O, 0grad, 20h;
|

21-acetoxy-9-bromo-11β,17-dihydroxy-pregn-4-ene-3,20-dione


chromium(III) chloride


zinc


hydrocortisone acetate
| Conditions | Yield |
|---|---|
|
With
acetic acid;
In
methanol; dichloromethane; water; N,N-dimethyl-formamide; acetone;
|
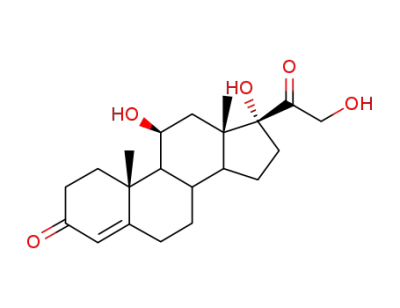
cortisol

acetic anhydride

21-acetoxy-9-bromo-11β,17-dihydroxy-pregn-4-ene-3,20-dione

chromium(III) chloride
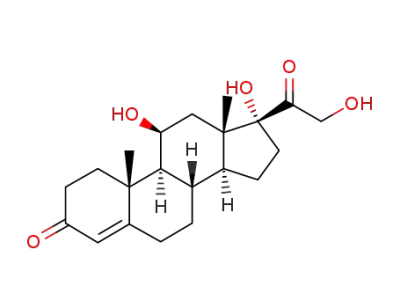
HYDROCORTISONE

Cortisone acetate

2α,11β,17α,21-tetrahydroxy-pregn-4-en-3,20-dion
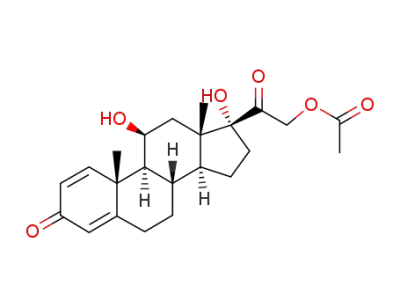
prednisolone 21-acetate
CAS:17949-65-4
CAS:14639-25-9
CAS:6217-54-5
CAS:6359-82-6